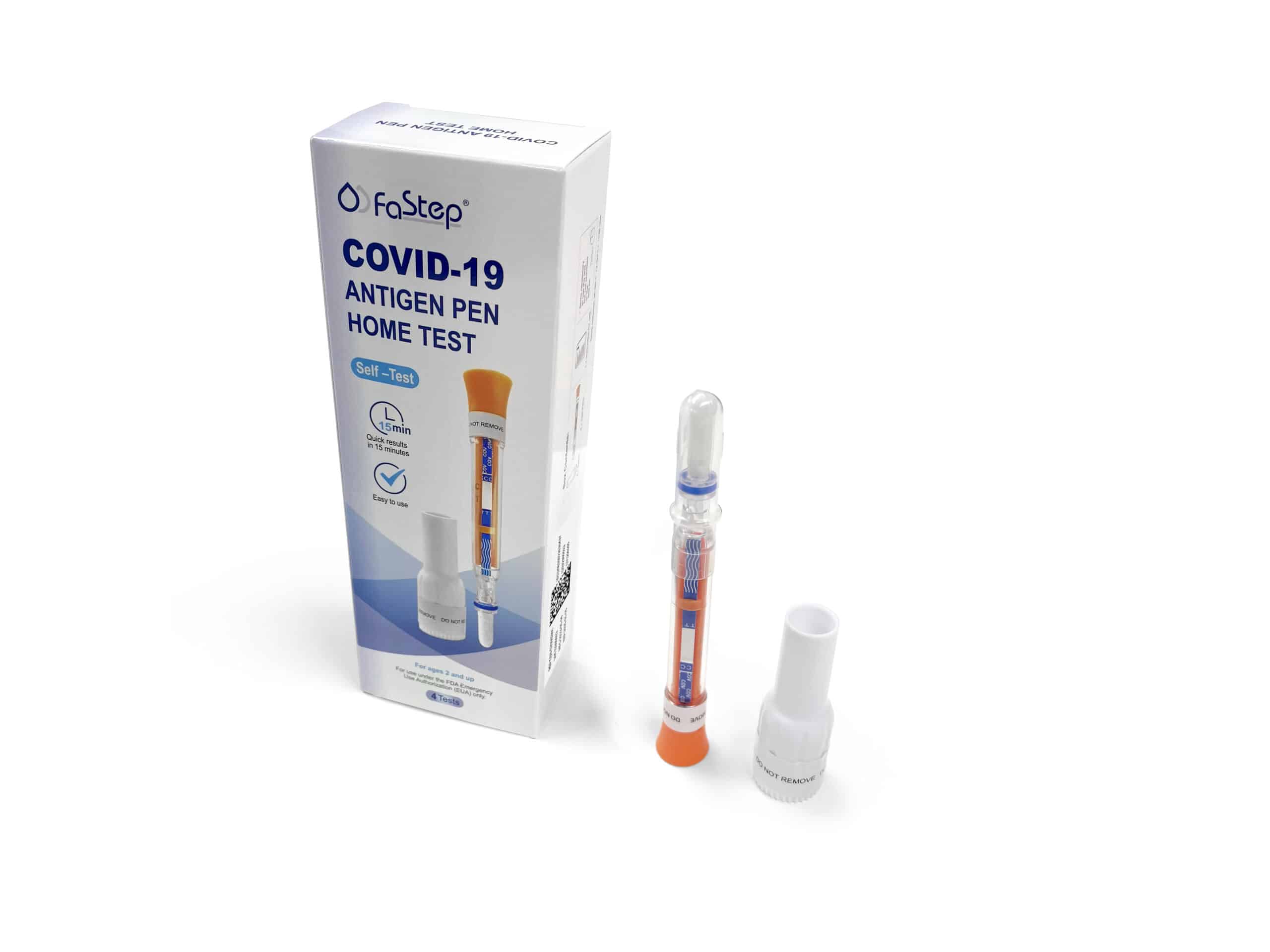
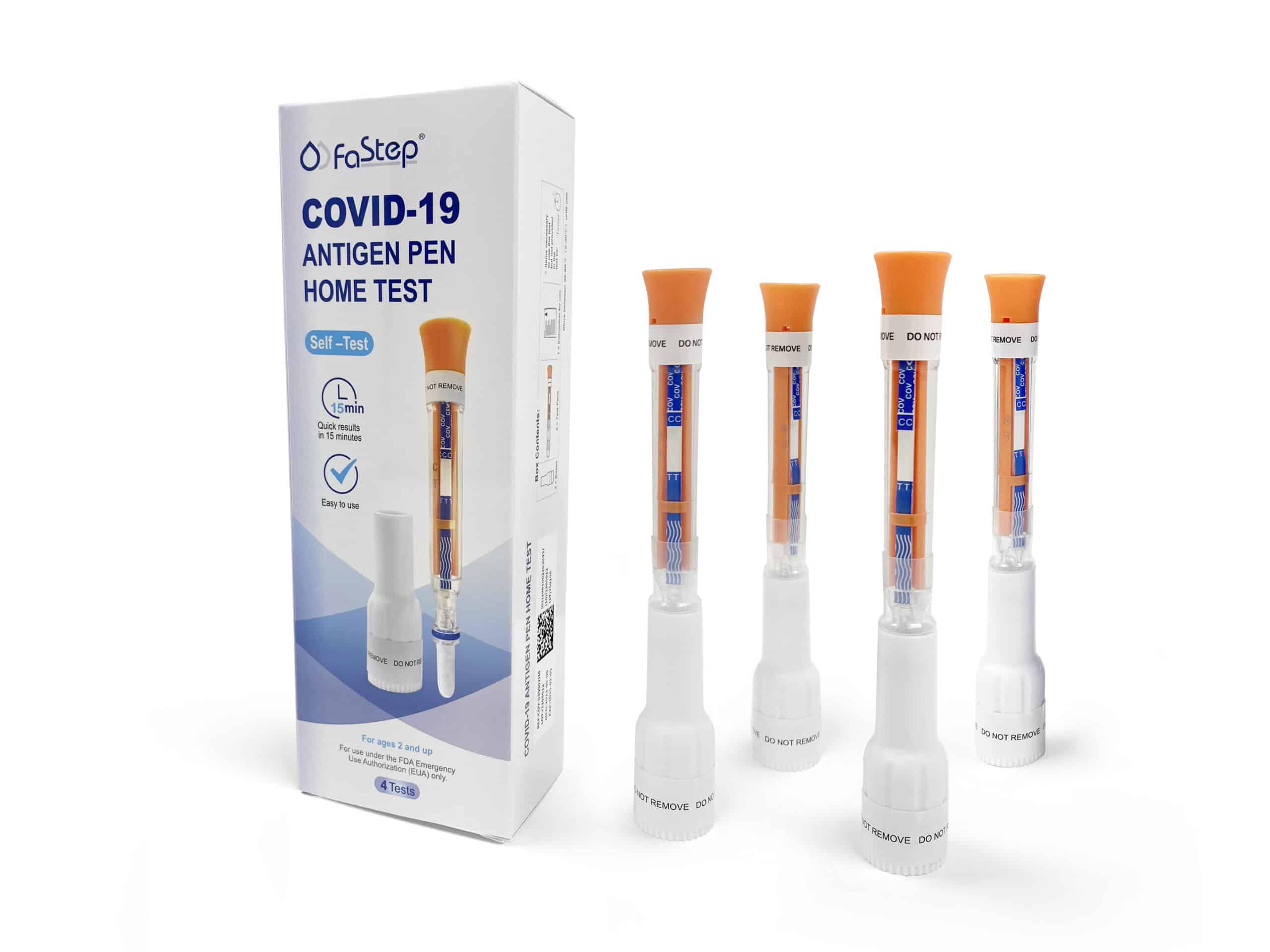
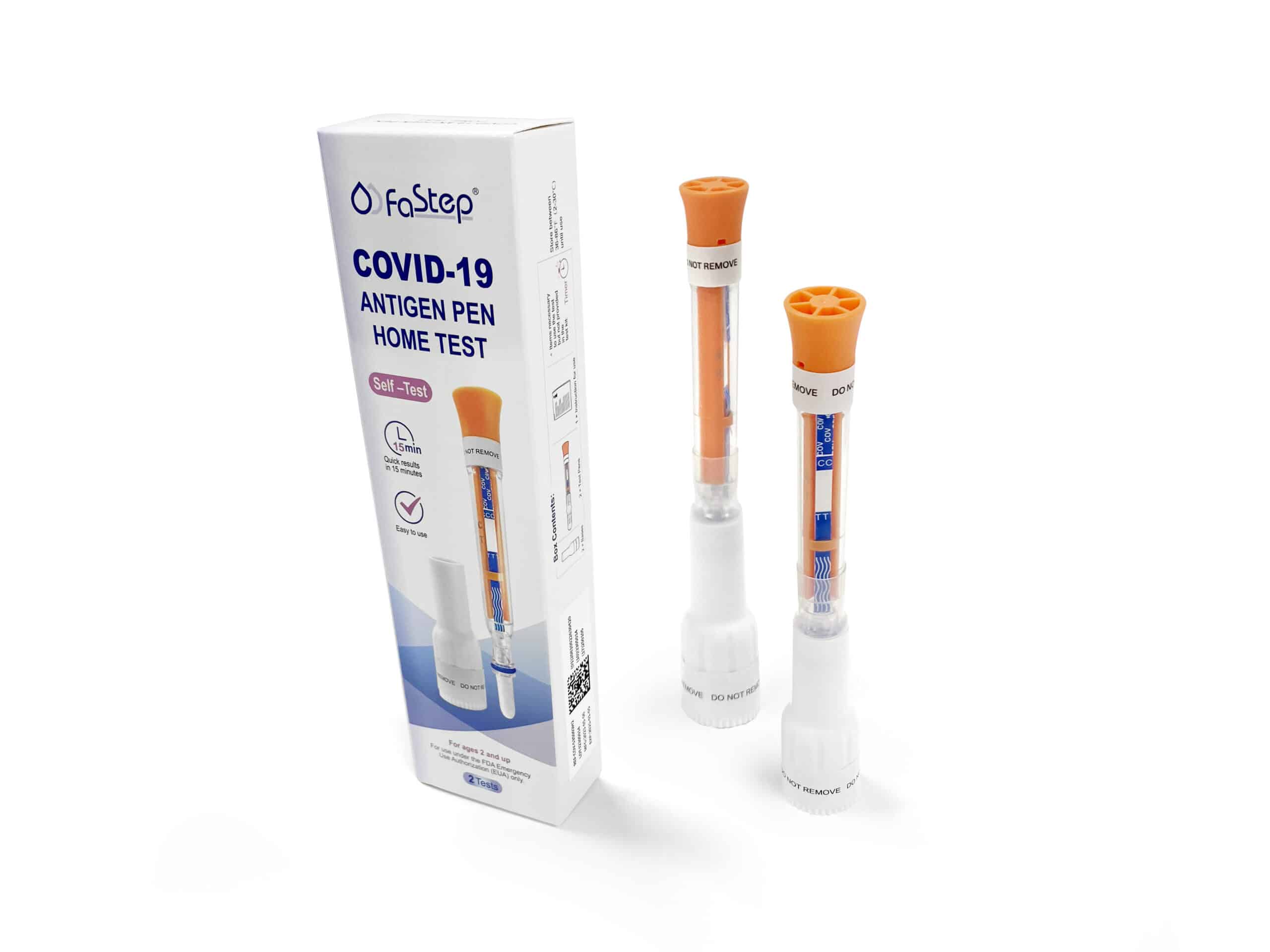
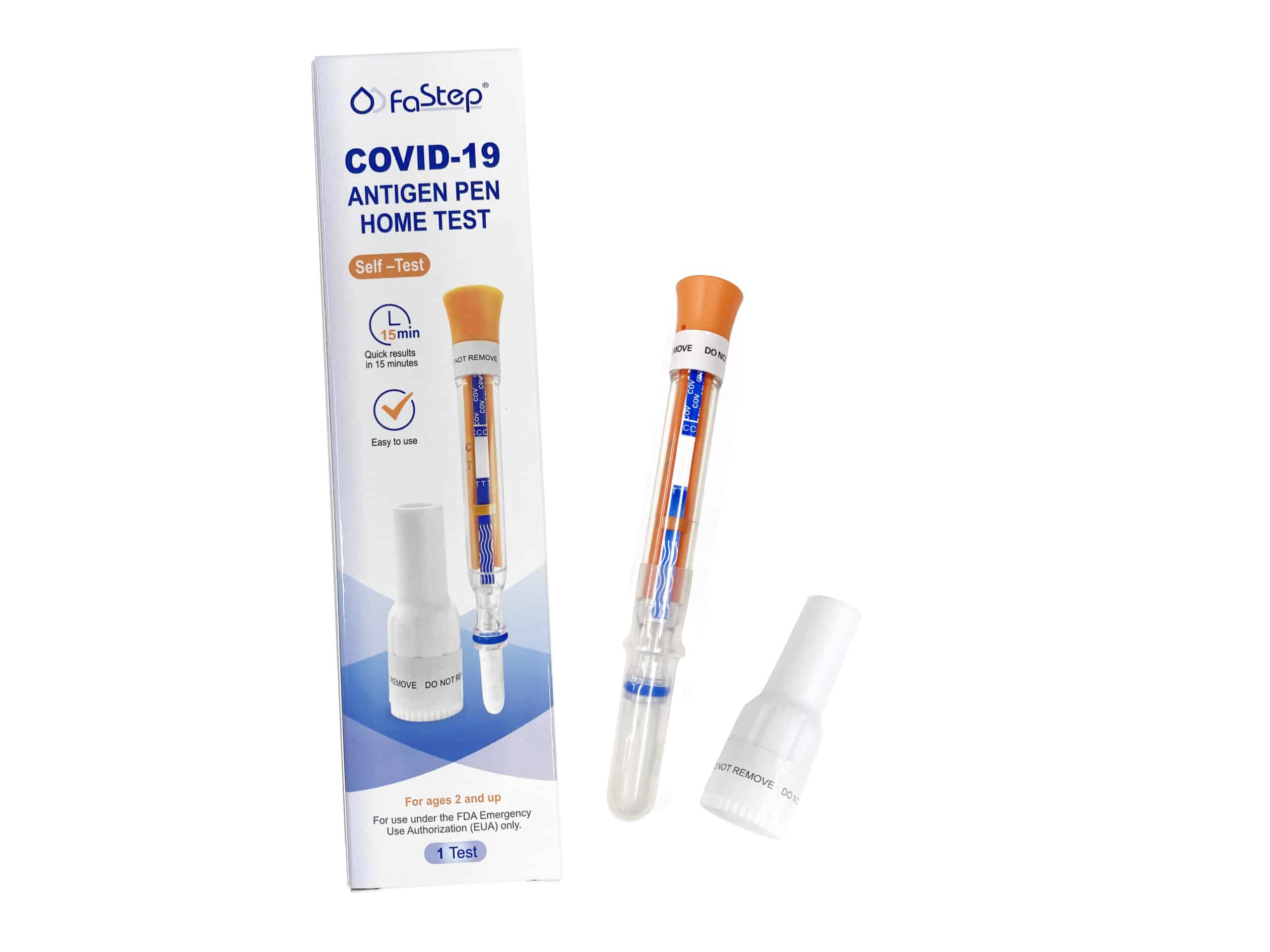
Instant Covid Test – Antigen Pen Home Test
$2.15
As low as $1.49
| Covid test | |
| Covid-19 Antigen Pen | |
Authorized by FDA under an Emergency Use Authorization (EUA)
Short Expiration date SALE, EXPIRE End of June 25
FREE SHIPPING 100 Covid tests or more, same day shipping
Choose quantity option
| Quantity | Price per test |
Orders will only be shipped on business days before 3.00 pm EST. Friday overnight delivery will only be received on Monday.
Intended use
The COVID-19 Antigen Pen Home Test is a lateral flow immunoassay device intended for the qualitative detection of nucleocapsid protein antigen from the SARS‑CoV‑2 virus.
This instant covid test is authorized for non‑prescription home use with self‑collected anterior nasal (nares) swab samples from individuals aged 14 years or older or adult collected anterior nasal (nares) swab samples from individuals
aged two (2) years or older. This test is authorized for individuals with symptoms of COVID-19 within the first 6 days of symptom onset when tested at least twice over three days with at least 48 hours between tests, and for individuals without symptoms or other epidemiological reasons to suspect COVID‑19, when tested at least three times over five days with at least 48 hours between tests.
The COVID‑19 Antigen Pen Home Test does not differentiate between SARS‑CoV and SARS‑CoV‑2. Results are for the identification of SARS-CoV-2 nucleocapsid protein antigen, which is generally detectable in anterior nasal (nares) swab specimens during the acute phase of infection. Positive results indicate the presence of viral antigens, but clinical correlation with past medical history and other diagnostic information is necessary to determine infection status. Positive results do not rule out bacterial infection or co‑infection with other viruses.The agent detected may not be the definitive cause of disease. Individuals who test positive with the Instant Covid Test – COVID‑19 Antigen Pen Home Test should self‑isolate and seek follow‑up care with their physician or healthcare provider as additional testing may be necessary.
All negative results are presumptive and confirmation with a molecular assay, if necessary for patient management, may be performed. Negative results do not rule out SARS‑CoV‑2 infection and should not be used
as the sole basis for treatment or patient management decisions, including infection control measures such as isolating from others and wearing masks. Negative results should be considered in the context of an individual’s recent exposures, history, and the presence of clinical signs and symptoms consistent with COVID‑19.
The COVID‑19 Antigen Pen Home Test is intended for non‑prescription self‑use and/or as applicable, for an adult lay user testing another person 2 years of age or older in a non‑laboratory setting. The COVID‑19 Antigen Pen Home Test is only for in vitro diagnostic use under the Food and Drug Administration’s Emergency Use Authorization. This product has not been FDA cleared or approved.
How to Use
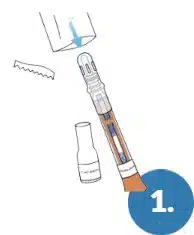
Remove the Test Pen and the Base from the packaging.
USE THE TEST WITHIN 1 HOUR OF OPENING.
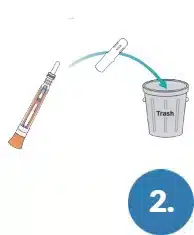
Remove Swab Cap from the Test Pen.
Discard the Swab Cap.
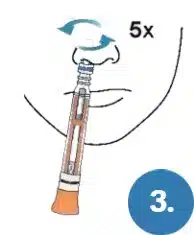
Gently insert the Swab end of the Pen in one nostril about 1/2 – 3/4 of an inch.
Firmly rub the swab at least 5 times against the inside walls of the nostril in a circular motion. Do not just spin the swab.
Make sure you have removed the swab cap (see step 2).
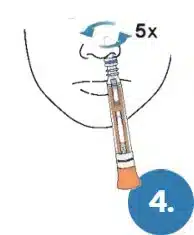
Repeat the process with the same swab in the other nostril.
Did you swab both nostrils? If not, inaccurate results can occur.
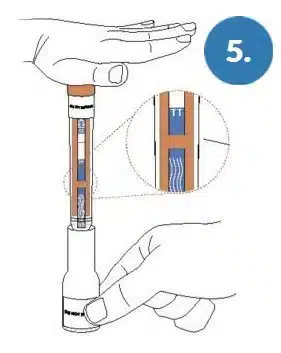
Place the Base on a flat surface. Place the swab end of the Pen into the base.
Locate orange band on the pen.
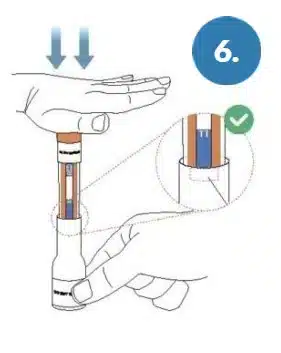
Press firmly on the test pen to insert it all the way into the base so that the orange band is completely covered by the base.
Orange Band Covered.
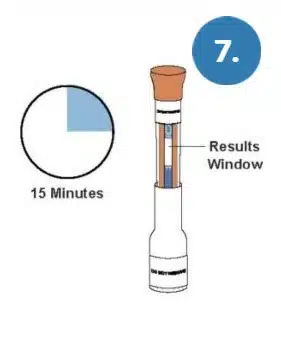
Leaving the test upright, set a timer and read the results at 15 minutes.
WARNING: Do not read results earlier than 15 minutes. Do not read the results after 30 minutes.
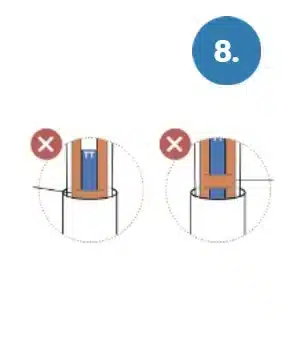
Orange band is still partially visible.
Orange band is still completely visible.
WARNING: Failure to insert the test pen all the
way into the base can lead to inaccurate results.
Reading the Results

Positive Result

Negative Result
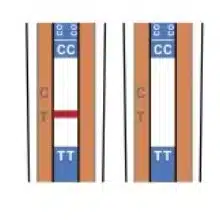
Invalid Result
| Weight | 0.8 lbs |
|---|---|
| Dimensions | 6 × 5 × 3.5 in |
Related products
Instant Covid Test Boson
As low as $1.75
| Covid test | |
| Nucleocapsid Protein Antigen | |
Authorized by FDA under an Emergency Use Authorization (EUA)
IN STOCK NOW: SAME DAY SHIPPING: LARGER ORDERS AVAILABLE
FREE SHIPPING 100 Covid tests or more, same day shipping
Choose quantity option
| Quantity | Price per test |



Reviews
There are no reviews yet.